DIESEL ENGINES- NEW & RECONDITIONED

HEAVY EQUIPMENT, PARTS & CONSUMABLES
WHAT OUR CUSTOMERS SAY
BLOG
What’s trending within Industrial Manufacturing Industries?
6 Cycliner Perkins engines
November 30, 20216 Cylinder The 6 cylinder option in the Perkins® 1100 Series, the 1106 range, delivers the power you need for earth moving...
Cummins Marine Featured Engines 2021
November 29, 2021When you think of a machine powered by Cummins, our engines working hand-in-hand with wind power to move a vessel is likely...
A Guide to Crankshaft for Equipment Engines
September 30, 2021The crankshaft is a moving part of the internal combustion engine (ICE). It’s main function is to transform the linear...
Top Marine Diesel Engines Leading the Marine Industry
September 22, 2021Marine power continues to change boats and boatbuilding. In this blog today we just want to list of some of the best marine...
Cummins Industrial Engine Series
August 11, 2021IZIPART is your one stop shop for a complete range of OEM and aftermarket Cummins Diesel Engines parts and Equipment. The...
TK-Series Tooth System | John Deere Construction Parts and Attachments
August 9, 2021Engineered for maximum strength and impact absorption, this breakthrough design delivers speedy installation and pin...
Tobacco Knives, Garniture Tape and Carbide Tools
August 4, 2021Whether you’re an end user or original equipment manufacturer (OEM), IZIPART will work with you to deliver the most efficient...
400 Series | PERKINS
July 27, 2021Perkins 400 Series is a proven family of engines designed to give optimised performance and robust technology for the...
CAT C7 Engines: Applications, Replacement Options and Parts
July 15, 2021The Cat C7 Engine has a minimum power output of 168.0 bkW, and maximum power output of 224.0 bkW along with a rated speed of...
Construction Equipment Attachments and Accessories
July 8, 2021Pairing an attachment with the proper excavator or wheel loader can help you adjust to changing market conditions, replace...
Hauni’s latest Tobacco Machinery and Technology
May 18, 2021Hauni continue to launch new and improved tobacco machinery and technology to serve the industry. In this blog, we talk about...
Cummins Diesel Engines Industrial Uses and Engines
April 28, 2021Cummins manufactures high-performance diesel engines for industrial uses including agriculture, construction, rail, mining,...
Types of Tobacco Machinery Consumable Products
April 21, 2021IZIPART offers multiple types of tobacco machinery products for brands such as hauni, molins, GD, focke, decoulfe, sasib and...
How to find your Engine Nº Location for Perkins
March 9, 2021On this guide we demonstrate how to find and locate your engine number on you Perkins Engine. The categories are divided...
Cigarette manufacturing use of Garniture Tape
February 23, 2021Any modern machine for making cigarettes and filter rods at high speeds requires the use of high quality tapes for good...
Perkins Engine application in Construction, Agriculture and Marine Industries.
February 10, 2021With an engine range from .5 to 36 litres, Perkins engines power more than five thousand different applications throughout...
Types & Usefulness of Carbide Insert Knives
January 26, 2021As we are aware that tungsten carbide is a chemical compound containing same ratios of tungsten and carbon atoms. In other...

A Simple Guide to Crankshafts
January 19, 2021The crankshaft is a moving part of the internal combustion engine (ICE). It’s main function is to transform the linear...
GD Tobacco making & packing machine spare parts
January 12, 2021Equipment Modernization G.D s.p.A portfolio of solutions enhances the value of operating equipment by installing the...
Updates made to the John Deere 310SL Backhoe
January 5, 2021To cater to the demands of customers seeking more productivity and efficiency out of their machines, John Deere has rolled...
Top Caterpillar Equipment & Their Uses
December 7, 2020Popular Caterpillar Equipment & Their Uses When it comes to construction machinery, the big yellow Caterpillar...
Contract Manufacturing: Cigarette OEM
November 24, 2020Maximizing production capacities as much as possible is important for investment-heavy cigarette manufacturers; and OEM is a...
Common Types of Mining Equipment: A guide to the Mining Industry
October 28, 2020Each type of mining equipment comes with its own set of mining activities. The most common types of mining equipment vary...
Buy new Doosan heavy Equipment? Or Replace with genuine doosan parts?
June 17, 2020We advise that before you rush into a decision of committing to purchase new Doosan Equipment because your Doosan equipment...
Benefits of Used Heavy Equipment – CAT/Doosan/Dressta/Komatsu
May 20, 2020Used construction equipment is increasingly becoming the purchase option of choice when Companies plan to replace or...
Reconditioned / Remanufactured Diesel Engines Explained (CAT, Cummins, Komatsu etc.)
May 6, 2020Now, many owners of Diesel Engines within heavy equipment Industries such as Construction, Truck &...
Easy tips to keep your Industrial Diesel Engine in ‘tip top’ Condition
May 3, 2020Maintaining your Diesel Engine is essential to ensure it can run optimally, increase its longevity, and reduce...
Что такое форматные ленты?
April 20, 2020Что такое форматные ленты? Форматные kленты используются в машинах для изготовления сигарет и фильтров и для...
COVID-19 is Contaminating the Sustainability of Supply Chains
April 5, 2020The recent COVID-19 outbreak has popularised the term ‘supply chain management’ due to the unprecedented, rapid and...
Guidance for Tobacco Manufacturers- Mitigating Supply risks from COVID 19
March 16, 2020Mitigating Supply risks from COVID 19 Weathering the storm For each product group Identify locations of Tier 1...
Sigara Üretimi İçin Doğru Garniture Bant Nasıl Seçilir
February 25, 2020Garnitür Bantları Nedir? Garnitür bantları Sigara & Filtre Yapım makinelerinde form verme amacıyla...

How to choose the correct Garniture Tape for Cigarette Manufacturing
February 10, 2020What are Garniture tapes? Garniture tapes are used in Cigarette & Filter Making machines and for tobacco...
Reinventing Manufacturing in 2020
February 2, 2020Generally, 2009 to 2019 were 10 golden years for manufacturers worldwide. After the swift recovery from the economic...

The role of ‘Empty Cigarette Tubes’ in Cigarette Manufacturing
January 27, 2020The cigarette tube consists of cigarette paper, which is usually pre-wrapped with an acetate or paper filter. It looks like a...
Why every manufacturer should have a Tobacco Reclaimer Machine!
January 20, 2020Tobacco Reclaiming Machine Benefits High yield with low degradation Very low paper count in recovered tobacco No...
Optimize filter quality via Hauni’s new Spray Nozzle Application
October 20, 2019The conventional cigarette is in decline on the world market. Therefore, product quality is one mayor factor for successful...
The Impact of Maintenance Operations On Supply Chain Management
February 14, 2019We all know how delays in any part of the supply chain can cause significant problems and incur additional delays among the...
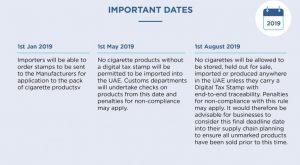
UAE Digital Tax Stamps on Tobacco Products
January 7, 2019FTA Introduce “Digital Tax Stamp (DTS)” has been introduced in the UAE by the FTA and is applicable for all...
9 Types of Maintenance: How to Choose the Right Maintenance Strategy
June 1, 2017Across industry, many definitions are used when it comes to the different types of maintenance. It can quickly get confusing...